Navigation auf uzh.ch
Navigation auf uzh.ch

The amyloid precursor protein (APP) is central to Alzheimer’s disease (AD). This single-pass transmembrane protein undergoes sequential proteolytic processing along two opposing pathways, with many of the resulting fragments having biological activity. In the non-amyloidogenic pathway, the neuroprotective secreted extracellular domain (sAPPa) is liberated by α-secretase, while the Ab peptide is destroyed. Amyloidogenic cleavage by b-secretase—followed by intramembraneous g-secretase cleavage—generates the Ab peptide that aggregates to form oligomers that have a suppressive effect on synapses and are deposited in amyloid plaques. Cleavage by g-secretase after a- or b-cleavage also releases the APP intracellular domain (AICD) from the membrane.
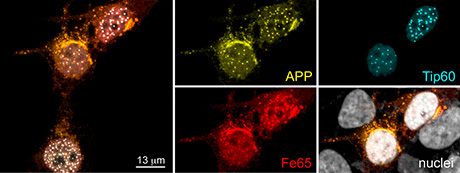
We could show that AICD translocates to the nucleus when bound to the cytosolic adaptor protein Fe65, where, together with the lysine acetyltransferase Tip60, they form AFT complexes that localize to spherical nuclear spots (see figure). We have characterized these AFT complexes and shown that they localize at the nuclear loci of AICD-regulated genes—among other evidence—and postulated that they represent transcription factories. APP thus has the capacity for nuclear signaling and we have further demonstrated that this property is mediated by the amyloidogenic—disease-causing—cleavage pathway of APP. Altered AICD nuclear signaling could therefore be involved in the molecular cascades leading to neuronal death in AD. We are now striving to better understand the regulation of AICD signaling by posttranslational modifications, adaptor proteins and degradation pathways. We have further established viral expression vectors and are studying the effects of AICD on neuronal and synaptic morphology and function. Finally, we are characterizing a set of AICD-regulated genes that function in synaptic biology. A better understanding of the physiologic function of APP will inform us about the effects on synapses and might identify novel therapeutic targets to prevent synapse loss and neuronal death in AD.
Research Technicians
Larissa Frei
Maik Krüger
Daniel Schuppli